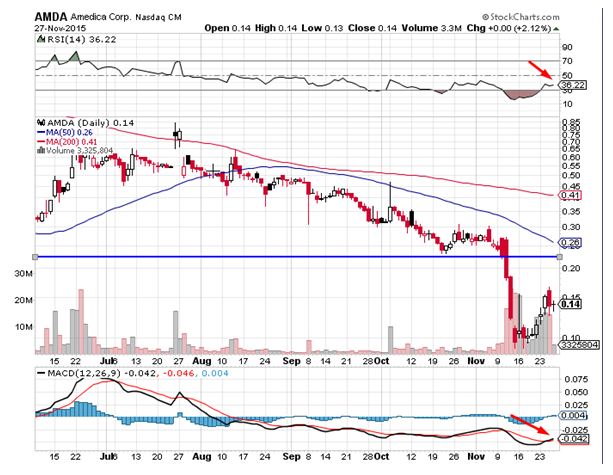
Amedica Corporation (NASDAQ:AMDA) was a notable mover during Friday’s trading session. The stock surged by close to 2% on the back of high volumes. The stock has been trending lower over the last couple of months underperforming the broader indices. The stock currently trades below all daily moving averages. Traders believe the move on Friday was a profit taking move. The momentum oscillators continue to trend in the oversold territory indicative of the strong selling momentum. The relative strength index is showing signs of weakness, which is bearish. Traders believe the stock could head to levels of $0.117 in the near term.
Amid improving market conditions, Amedica Corporation (NASDAQ:AMDA) announced its financial results for the recently finished 3Q2015. As per the reports, the cash burn level of the company came down by $4.4 million or 38% during the third quarter.
Amedica is an innovative biometric company, which is known for developing silicon nitride ceramics that can be used as a platform for biomedical applications.
3Q2015 Highlights
The cash and cash equivalents at the end of the quarter were $11.3 million. In terms of operative growth, Amedica had to use a significant portion of cash in different operations, which was 43% or $1.3 million higher than the cash used in operations during 3Q2014. It put a lot of efforts to balance its debt position, as a result of which, it managed to reduce the principal debt amount by 14% or $3.5 million.
The cost of revenues during the quarter came down by 15% due to reduced sales. However, the gross margin for the quarter was 73% of total sales compared to 84% during the same period in 2014. Operating expenses declined by $3.7 million or 38% during the quarter. Amedica ended the quarter with a net loss of $10.1 million.
To make sure that investors don’t have to face any trouble, it removed conversion feature from its convertible notes.
Amedica submitted the performance data of CASCADE clinical trial spanning over last two years to U.S. Food & Drugs Administration. It expects to receive a response from the regulator in 1Q2016. The efforts regarding the 1st generation silicon nitride interbody instrumentation and devices that Amedica had been putting for a long time paid off as it got the clearance from the Brazilian government. Soon after this clearance, Amedica shipped the first lot to Brazil without any delay.
It doesn’t want to leave any stone unturned when it comes to initiating innovations. During the September quarter, it appointed one of the most renowned and sought after scientists, Giuseppe Pezzotti, Ph.D., to its Scientific Advisory Board. Further details about such changes will be made public from time to time.