ActiveMarketInsider.com initiated coverage on StemCells Inc (NASDAQ:STEM) that sent the stock prices of STEM through the roof for a staggering 500 percent gains. However, the real reason for today price action was a press release issued by the company. The company announced that it would merge with Israeli private company Microbot Medical Ltd. to develop robotics-based medical devices. As per the press release, Ian Massey, the CEO of StemCells, Inc stated that “This transaction concludes an extensive search for strategic alternatives conducted by StemCells since we failed to see robust clinical results in our Phase II clinical study of human neural stem cells in chronic spinal cord injury. We believe both our investors and the market at large will see the potential of Microbot’s robotics platform, specifically its catheter and shunt technologies, and will appreciate Microbot’s overall business opportunities and potential.”
StemCells Inc (NASDAQ:STEM) opened at $0.45 and so far has hit a high of $2.99 for over 500 percent gains in just a day. As per the press release, Ropes & Gray LLP acted as legal advisor to StemCells and Ruskin Moscou Faltischek, P.C. and Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. acted as legal advisor to Microbot.
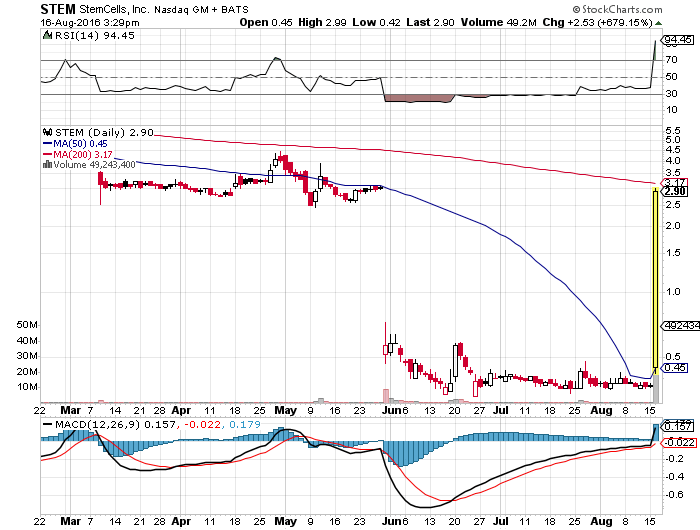
The terms of the merge agreement were unanimously approved by the board of directors of each company. However, it is important to note that then completion of the merger is subject to approval of the StemCells and Microbot shareholders and certain regulatory approvals and customary conditions. Looks like StemCells will be negotiating reductions in outstanding balances with its creditors that might help its balance sheet.
For the uninitiated, just 3 months before the company had announced that it will terminate the Company’s Phase II Pathway Study in spinal cord injury following an in-depth review of data from the study and after obtaining the concurrence of the study’s Interim Analysis Data Monitoring Committee (the “IA-DMC”). While the results showed overall improvement in patients treated with the Company’s proprietary cells, the magnitude of the effect and the perceived trend of the effect over time did not justify continuing the study or exploring the variability in the initial patient observations, given the financial resources available to the Company.
We will be updating our subscribers as soon as we know more. For the latest updates on StemCells Inc (NASDAQ:STEM) singup for our newsletter.


