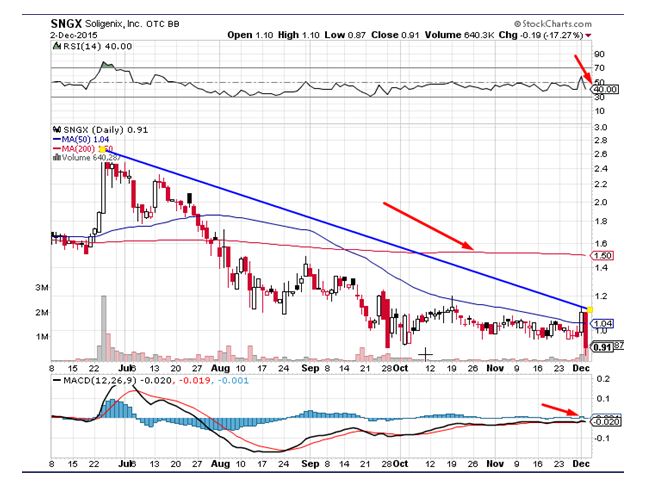
Soligenix Inc (OTCBB:SNGX) was notable decliners during Wednesday’s trading session declining by 17% on heavy volumes, which were 10 times the daily average. The stock has been underperforming the broader markets over the last several months. Soligenix has been forming lower tops and lower bottoms indicative of the strong bearish momentum and hit a fresh 52-week low on Wednesday. The oscillators, which measure momentum, are clearly indicating that bears are in total control at the momentum. The stock trades below all daily moving averages. The index for relative strength has given a fresh sell signal, which is a bearish sign.
Soligenix Inc (OTCBB:SNGX), better known for developing medical treatments to cure rare diseases that require utmost attention, announced its financial and non-financial highlights for 3Q2015. As per the reports, its revenues for the quarter were accounted $3.9 million compared to $2.9 million in 3Q2014.
Other Financial Highlights Of The Quarter
If taken into consideration the performance of Soligenix during the first nine months of 2015, one can see that it managed to generate a total of $5.8 million in revenues as compared to $5.1 million in 2014. Two major contributors in this revenue surge were RiVax and OrbeShield government contracts.
Its net income for the September quarter was $0.10 per share or $2.8 million in total as compared to $(4.3) million during the same three-month period in 2014. For the first nine months ending September, the net income was $(5.8) million compared to previous year’s $(8.6) million. This change was primarily driven because of change in the fair value of the warrant liability of the warrants issued by the company as part of its recent public offering.
Soligenix managed to bring down its expenses during the quarter on the back of excellent financial planning. Research and development expenses for the quarter were $1.3 million against $5.1 million during 3Q2015. For the first nine months, the R&D expenses were $3.7 million as compared to $7.3 million during the first nine months of 2014.
Reports claim that the company acquired synthetic hypericin product candidate named SGX301 from Hy BioPharma for as much as $4.0 million. This amount was charged to R&D expenses during 3Q2014, which led to an increment in R&D expenses in the last year.
Soligenix ended the quarter with as much as $4 million in the form of cash and cash equivalents. It’s committed to improving the financial position in the coming quarters by taking several first-time initiatives, details of which will be announced at a later date.