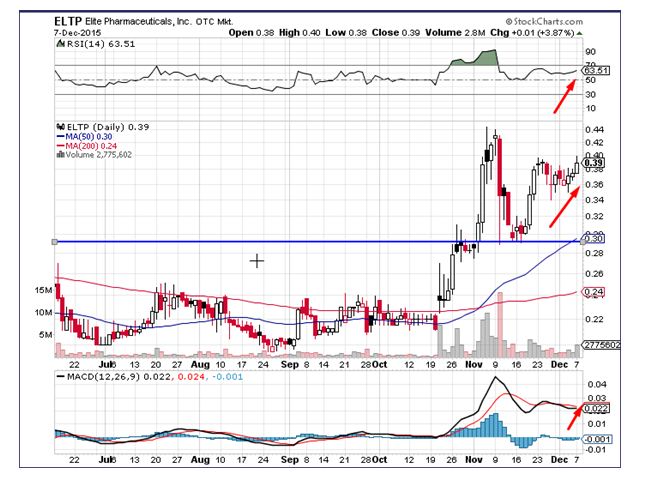
Elite Pharmaceuticals Inc (OTCMKTS:ELTP) was a notable mover during Monday’s trading session rallying by 4% on above average volumes, which were 0.9 times the average turnover. The stock has formed a strong support at levels of $0.293 and is showing signs of retesting the resistance zone at $0.436. Elite Pharmaceuticals currently trades above all important moving averages, which have formed a golden cross. The index measuring relative strength has given a fresh buy signal, which is a positive. Momentum indicators are clearly pointing towards a bullish reversal. Traders see the stock heading towards levels of $0.42 in the intermediate term.
Elite Pharmaceuticals Inc (OTCMKTS:ELTP) released financial report for the quarter ended September 30, 2015. The company reported that consolidated revenue for 2Q2016 jumped 115% YOY to $2.7 million. This remarkable jump in revenues can be attributed to the continued growth of company’s niche generic product lines. During 2Q2016, the pharmaceuticals entity also invested an additional $4.2 million in the advancement of ELI-200.
The details
In October 2015, Elite reported positive top-line data from the Phase III pivotal study of its lead abuse-deterrent opioid, popularly known as ELI-200, for the cure of moderate to severe pain. The management reported that the company is on schedule to submit a New Drug Application with the Food and Drug Administration in this year. Elite will release an update of advancement activities in the coming days.
Nasrat Hakim, the CEO and President of Elite, reported that it was another remarkable quarter. The generic products recorded another revenue milestone, and they successfully concluded Phase III studies for ELI-200. Elite has been making substantial progress as it move forwards towards the submission of NDA for ELI-200 this year and the planned drug launch in 2016.
Abuse deterrent technology
Elite reported that its proprietary abuse deterrent technology, known as ART™, comes in the list of multi-particulate capsules. These capsules contains an opioid agonist apart from naltrexone, an opioid antagonist utilized largely in the management of opioid dependence and alcohol dependence. When this medication is taken as advised, the naltrexone is set to pass through the human body unreleased.
At the same time, the opioid agonist is released as intended offering therapeutic pain relief for which it is recommended.If the multi-particulate capsules are dissolved or crushed, the opioid antagonist is set to release and hence block the impact of active opioid agonist. The naltrexone absorption is set to block the euphoria and thus reducing the reason for misuse or abuse by recreational drug abusers.