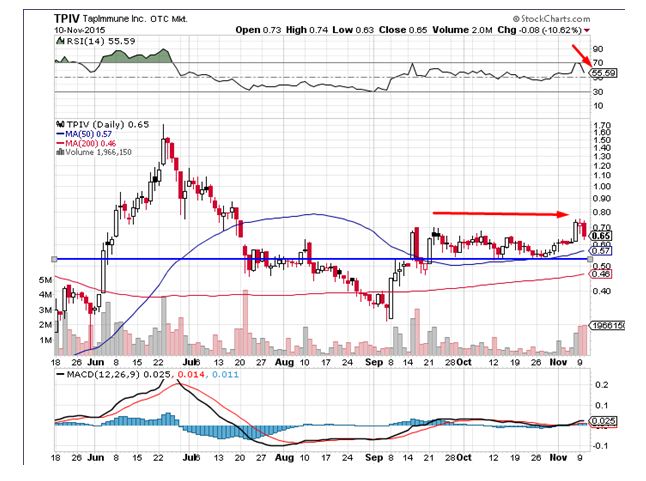
TapImmune Inc. (OTCMKTS:TPIV) was a massive decliner in trade in the overnight session. The stock plummeted by close to 11% on the back of 21 times the average daily volumes indicative of the shift of momentum towards the sell side. The stock has been trading in a broad trading range over the past few weeks indicative of the lack of direction. The stock currently trades above all important daily moving averages. The relative strength index for the stock has given a sell signal, which is a bearish signal. Traders believe the stock could head to levels of $0.57 in the near term.
TapImmune Inc. (OTCMKTS:TPIV) vaccine is all set to be studied by Mayo Clinic after it has received a whopping $13.3 million grant from U.S. Department of Defense. The grant will be used to cover the cost of Folate Receptor Alpha Vaccine Phase II Clinical Trial covering 280 patients suffering from Triple Negative Breast Cancer.
Insights of The Matter
The grant has been rewarded to Keith L. Knutson. Ph.D. and Edith A. Perez, M.D. Knuston is the professor of Immunology at the Mayo Clinic Center for Immunology and Immune Therapies, whereas Perez is the deputy director at large of Mayo Clinic Cancer Center. Both of them were joined by Frances C. Durling and Serene M., who teach Medicine at Mayo Clinic College of Medicine.
It’s not the first time when TapImmune has come with a vaccine like this. There were many occasions in the past when it developed vaccines that had a huge impact on human health. In this case, TapImmune reserves all the rights to commercialize this vaccine that can be used for the treatment of ovarian, endometrial, breast and non-small cell lung cancer.
TapImmune has been working for a very long time with Dr. Knutson regarding the development of Folate receptor alpha and HER2/neu vaccines. It’s a known fact that TapImmune is an expert in the clinical development field. As part of this project, it will work tirelessly with Mayo Clinic and provide manufacturing and clinical expertise. Apart from this, TapImmune will also provide correct GMP vaccine formulations to Mayo Clinic, so that the trial can be executed in an easy and hassle-free way.
To make sure that Phase II clinical programs in triple negative ovarian and breast cancer can be executed successfully, these vaccine formulations along with other immunotherapeutics need to be provided.
The senior management of the company, including CEO & Chairman Dr. Glynn Wilson, is delighted to make this announcement and hopes that things will continue to fall in line in the future as well.