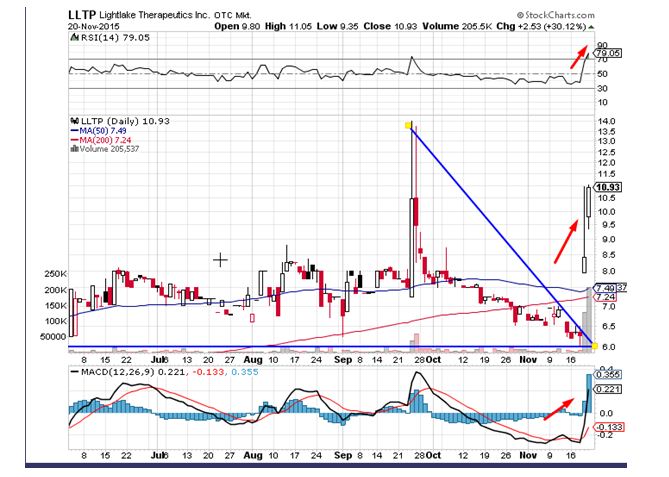
Lightlake Therapeutics (OTCMKTS:LLTP) was a massive mover in Friday’s trading session. The stock surged by close to 31% on the back of 20 times the average daily volumes. It is important to indicate that the stock has been in an uptrend over the last couple of weeks ever since hitting an intraday low of $6.02, which is being seen as a huge positive. The stock was able to break above all daily moving averages on the back of the price volume action seen over the past two trading sessions. Traders believe the stock could head to levels of $12.00 in the near term.
Lightlake Therapeutics (OTCMKTS:LLTP), better known for developing pharmacological treatments for eating, addictive disorders and substance orders, has received funding commitment. As per the reports, an international research and development foundation has agreed to provide funding to Lightlake.
Insights of Matter
The foundation that has agreed to provide funding is poised to provide different research based initiatives that primarily focus on health issues. Lightlake has been trying for a very long time to conduct studies that can advance indications, which address different health issues. Reports claim that the total funds that the foundation has agreed to deploy are $1.6 million, on which it will receive around 2.1% interest in the net profits earned by the company on its products.
It’s not the first time when Lightlake has received the funding commitment from the foundation. Previously, it committed around $3 million in July 2014 to advance the progress in opioid overdose reversal treatment, so that Lightlake can take FDA’s approval and launch the drug commercially. In return for this investment, Lightlake received 6% share in the net profit made through this treatment and 6% interest in overall treatment. All the terms and conditions of this deal were clearly mentioned in the funding commitment investment agreement.
About a year ago, in December 2014, Lightlake made public a licensing deal with Adapt Pharma Limited’s subsidiary. Under the terms of this deal, Lightlake could receive potential payment exceeding $55 million and double-digit royalty, in exchange of offering the license for opioid overdose reversal treatment.
Management Call
The senior management team of Lightlake is delighted to announce this update and hopes that things will continue to move in the desired way. According to Dr. Roger Crystal, Chief Executive Officer, Lightlake, this funding will create a solid base for the future critical treatments. It’s their confidence in the capabilities of Lightlake that has prompted the foundation to invest for the second time. Lightlake will look forward to living up to their expectations in the future as well.