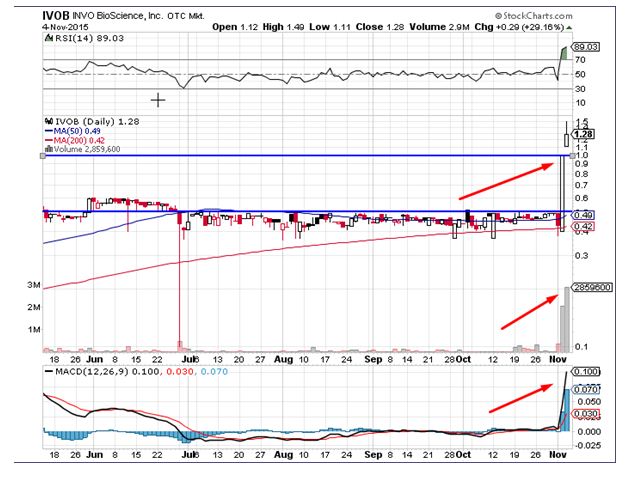
Invo Bioscience Inc (OTCMKTS:IVOB) was one of notable mover in the overnight trading session. The stock surged by close to 30% on the back of 20 times, which is considered to be a huge positive by traders. The stock currently trades above all important daily moving averages. The momentum indicators for the stock continue to trend in the bullish zone and show no signs of a reversal indicative of the clear shift of momentum towards the buy side. Traders believe the stock could head to levels of $1.5 in the near term and would find support near the levels of $0.997.
Invo Bioscience Inc (OTCMKTS:IVOB) has successfully received the FDA clearance for its INVOcell Fertility Treatment. Reports claim that Invo Bioscience has become the first intravaginal culture system company in the United States to receive clearance for this treatment.
Now, millions of infertile couples in the country will be able to take advantage of INVOcell fertility treatment without any hassle.
Management Call
The senior management team of Invo Bioscience is delighted to inform all the shareholders about this approval, hoping that it will transform the way infertile couples are treated in the country. According to Katie Karloff, CEO, Invo Bioscience, no other company has launched a similar product ever in the country, which makes Invo Bioscience as the sole marketer of fertility treatment in U.S.
The INVOcell fertility treatment can change the way in which medical professionals assist infertile couples to achieve good results in a more natural, simpler and cost-effective way. Invo had to fight a long battle with the health authorities in the country to receive the right to market and sell the INVOcell fertility treatment. Finally, FDA has given approval to country; thereby, opening a new road to treat infertility in an effective way.
After this approval, the next step is to find companies that can help Invo Bioscience reach to more and more patients across the country, said Karloff. According to a 2012 study conducted by World Health Organization, there’re more than 48.5 million couples across the globe suffering from infertility, of which 6.7 million belonged to U.S. Of these many couples, not more than 10% receive the exact treatment they need, making it an untapped market for the company.
INVOcell is mainly used for the incubation of sperm and eggs during early embryo development. It’s different from IVF technique and can bring faster results without putting huge money on the stake. Health experts consider it the need for the hour and forecast a sound market response ahead.