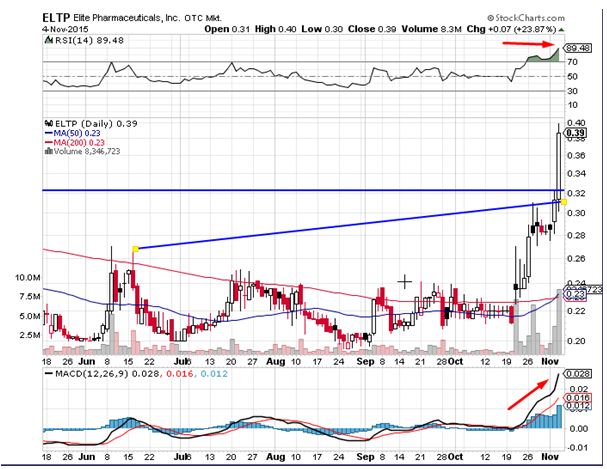
Elite Pharmaceuticals Inc (OTCMKTS:ELTP) was a massive mover in the overnight trading session. The stock surged by close to 24% on the back of 8 times average daily volumes considered to be a bullish sign. The stock currently trades above all important daily moving averages. The relative strength index for the stock continues to trend in the overbought zone but shows no signs of a reversal indicative of the strong buying interest present at current levels. Traders on the street believe the stock could head to levels of $0.47 in the near term and have formed a good support near the $0.32 level.
Elite Pharmaceuticals Inc (OTCMKTS:ELTP) announced top-line results from the phase 3 pivotal trial for its lead candidate ELI-200, which can be used to treat pain. As per the reports, the results are positive as the company has met its primary endpoint, which demonstrate the statistical significance that ELI-200 can be used for pain relief after surgeries.
All the secondary endpoint results that Elite Pharmaceuticals got, were in line with the primary findings and safety measures included in the study. No serious effect, allergy or death due to ELI-200 was recorded during the entire trial.
Road Ahead
Elite looks forward to submitting a New Drug Application to FDA for abuse-deterrent ELI-200 by the end of the year.
The senior management of the company is delighted to announce this news and hopes that it will have a positive impact on company’s offerings in the coming months. According to Nasrat Hakim, CEO & President, Elite Pharmaceuticals, it’s great to have finished the all-important first step in the commercialization of company’s very first abuse-deterrent product, ELI-200. It will pave the way for many more such developments in the coming months. Now, Elite is ready to take its next step i.e. submit NDA for ELI-200 to FDI by the end of 2015, he further added.
First Quarter FY2016 Revenues
Not long ago, Elite Pharmaceuticals announced financial results for the first quarter FY2016. As per the reports, the operating profit for the quarter totaled $2.5 million while consolidated revenues rose to $7.1 million. Elite invested a total of $2.4 million in clinical trials and other product development activities mainly focusing on ELI-200. Net income of the company in the first quarter was $16.1 million
The management team is delighted with these results and plan to continue its efforts to find out new lead candidates in the future as well. Further updates about Elite’s endeavors will be made public from time to time.