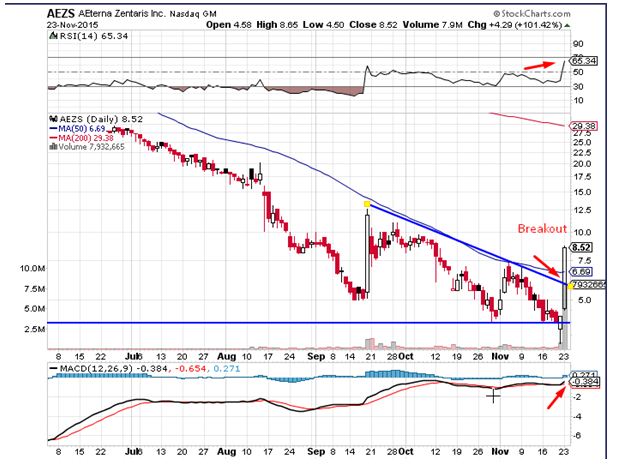
AEterna Zentaris Inc. (USA)(NASDAQ:AEZS) saw a massive up move during Monday’s trading session. The stock surged by close to 102% on the back of 30 times the average daily volumes. The stock broke out over the trend-line resistance on the back of the price volume action seen during the trading session, which is a bullish sign. The momentum oscillators for the stock have given a buy signal pointing towards a return of buying interest. Traders believe the stock could head to levels of $10.10 in the near term. The relative strength index saw a massive move indicative of inherent strength, which is a bullish sign.
AEterna Zentaris Inc. (USA) (NASDAQ:AEZS) reported that the company has enrolled first patient for its confirmatory Phase III clinical trial to show the efficacy of Macrilen™, a unique ‘orally-active’ ghrelin agonist for application in assessing adult growth hormone deficiency.
David A. Dodd, the CEO, Chairman and President stated that they remain committed to the advancement of Macrilen™ because of their confidence in its safety and efficacy, as well as the medical demand for such an appropriate assessment in the absence of an FDA-permitted diagnostic evaluation for AGHD. Here, it should be noted that AGHD affects nearly 75,000 adults across Europe, Canada and the US.
The study
AEterna reported that the confirmatory Phase III clinical trial of Macrilen™, entitled “Confirmatory validation of oral-macimorelin as a GH stimulation test for the diagnosis of AGHD in comparison with the ITT”, is planned as a two-way crossover trial with the insulin tolerance evaluation as the benchmark comparator. The trial will include some thirty sites in Europe and the United States.
The trial population will comprise of about 110 subjects at least 55 ITT-negative and 55 ITT-positive with a medical history detailing risk elements for AGHD. AEterna reported that the trial will cover a spectrum of enrollments from those having a low probability of having AGHD to subjects with a high risk of getting the condition.
The details
The primary endpoint of the study will be validation of a single oral regimen of macimorelin for the diagnosis of adult growth hormone deficiency, utilizing the ‘ITT’ as a comparator. AEterna reported that the coordinating investigator will be Jose Manuel Garcia, MD, PhD. Based on meetings with the FDA and the European Medicines Agency and following written scientific advice, AEterna believes that the trial meets the EMA’s and the FDA’s trial-design expectations permitting European and US approval, if successful.