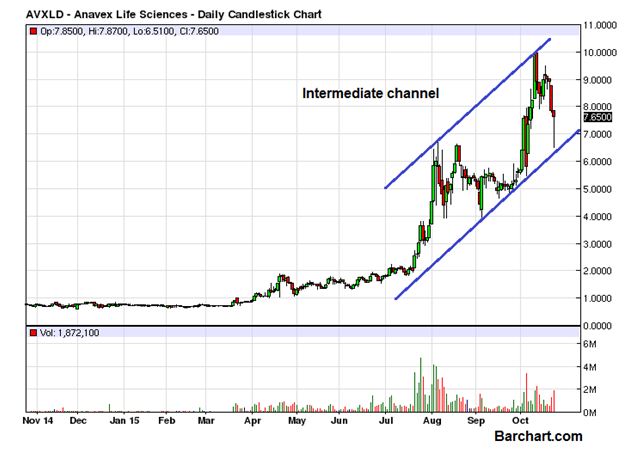
ANAVEX LIFE SCIENCES (OTCMKTS:AVXLD) continued its short term decline and finished the last trading session with a loss of 2.8% but the price action had something to encourage the bulls. The volume of day at 1.8 million was higher than the daily average of 1.1 million but the bigger part of that activity could be attributed to the bulls. The EOD loss of 2.8% doesn’t really reflect the 17% intraday recovery from the day low of $6.51, which has helped the daily candle to take the form of a huge Bullish Hammer. The bounce from the channel boundary adds to the strength of the bulls.
An article on ANAVEX LIFE SCIENCES (OTCMKTS:AVXLD) published on Seeking Alpha stated that the company is not some scam firm and boasts believable science. The company’s stock, in a matter of just three months, has gone up five-fold, making investors a bit of profit.
The highlights
In an interview with Dr Missling, the author has asked him some questions like the selection process deployed by the Michael J Fox foundation to allow grant, the details of clinical study of lead candidate Anavex 2-73, peer reviewed journal citations for the concept behind drug, competitive analysis of various elements used in trial and so on. The writer also asked him that did Anavex paid $200,000 to a penny stock outfit for a one day promotional campaign?
Coming to the last question, anyone who does not possess some benefit is going to pay such a hefty amount for a one day campaign and that too during an economic downturn. However, Anavex has strong results from its initial study to naturally get the attention of investors. The writer further states that even if the promotional news is true, it is ok as long as it is not about false promotion.
The grant
Unlike retail investors, MHFF have robust research process in assessing a grant request, and therefore they cannot be easily deceived. If they have allowed Anavex a grant, it suggests that they have evaluated the study and drug candidate, and are optimistic about its application in Parkinson’s.
ANAVEX 2-73 showcased early evidence of enhanced cognition in Alzheimer’s patients from the preliminary phase 2a trial data at the AAIC in July 2015. Late September, Anavex completed enrollment process for the Phase IIa Alzheimer’s study, registering 32 patients in all. This provides it ample time to report top-line data by end-2015. Although it is an MTD study, it could still serve as a major booster for company’s stock price.