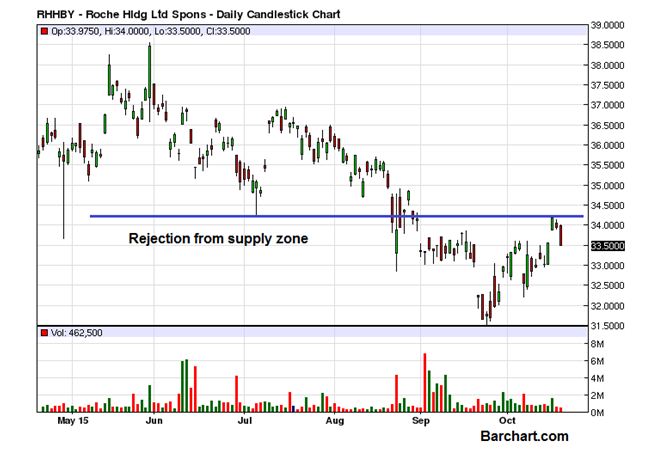
In last trading session, the stock price of Roche Holding Ltd. (ADR)(OTCMKTS:RHHBY) extended weekly drop and closed 1.33% in red. Back in September, the company reported the CD20 aiming antibody was the first experimental treatment to validate efficacy in a Phase III study with primary progressive patients. Its once-unsuccessful rheumatoid arthritis treatment hit a bulls eye in relapsing MS.
The details
Going into a latest scientific conference, the MS community already knew “Ocrelizumab” was a game-changer. Roche’s Ocrelizumab can be stated as an antibody drug that selectively lessens beta cells that convey the CD20 antigen on their shell. This CD20 antigen is mainly present on cells in the transitional phases of their advancement, which should enable for both retention and reconstitution of long-term immune memory.
Although this appears a bit of a stretch, this intermediate targeting can detail Roche’s Ocrelizumab’s remarkable tolerability level. During the Phase III Opera studies, which directly evaluated Ocrelizumab to Merck & Co., Inc.(NYSE:MRK)’s Rebif, a combined 88% of subjects getting Ocrelizumab treatment completed two years of treatment. It is a bit extended than the 82% of subjects completing 2 years of therapy with standard of care Merck’s Rebif.
The report
Talking about the efficacy, an even better sign of Roche’s experimental drug tolerability is that 97% of subjects agreed to remain on Ocrelizumab treatment after taking it for 2 years. A twice-yearly dosage regimen may have played an important role in patients’ decision. It was evident that enrollments were ready to stick to Roche’s Ocrelizumab. The drug’s efficacy compared to Rebif is even more alarming to ABCR market share. Patients getting the experimental treatment overwhelmed the standard of care in aspects of count of new brain lesions and disability progress.
Nearly 15% of MS patients can be stated as PPMS, and the rapid development of their disease creates immense problems to treat them.
Roche Holding Ltd. (ADR) (OTCMKTS:RHHBY) saw a range expansion yesterday as it ended the last trading session with a loss of 1.33%. it was not particularly a major decline but from the perspective of the previous narrow range days it was enjoying, the scale of the decline took a larger significance. The volume of the day at 462,000 was much lower than the daily average of 1 million but in a firm downtrend, the initial volume pattern often becomes misleading. The price is now close to the short term support area around $33.00-$33.50 but if a bounce doest materialize immediately, a much deeper decline can’t be ruled out.