Pharmacyte Biotech Inc (OTCMKTS:PMCB) reported that its upcoming clinical study will assess whether its pancreatic cancer treatment can meet a severe unmet medical need for people suffering with pancreatic cancer, particularly when the gold standard therapy is no longer effective in curing the disease. The treatment in question covers administration of Cell-in-a-Box® capsules in combination with low-doses of ifosfamide. As of now, there is no adequate alternative therapy available in the market.
The details
Translational Drug Development, along with Dr. Matthias Löhr and Dr. Manuel Hidalgo provided invaluable guidance and information to Pharmacyte in the planning of clinical study in advanced pancreatic cancer. This study intends to formulate an effective therapy for the large percentage of pancreatic cancer patients who fails to respond to the gold standard treatment.
There are few more treatment options, but marginally effective to treat advanced pancreatic cancer. Pharmacyte team is of view that their designed pancreatic cancer treatment, known as consolidation therapy could prove to be a turning point.
The treatment
As previously mentioned by Dr. Löhr in his recent publication on the unmet medical need being considered by Pharmacyte Biotech, chemotherapy may be effective in preventing relapse in patients whose tumors have been taken out surgically, but the occurrence ration of such cases is just 20%. The rest of the patients whose pancreatic cancer tumors are inoperable have no option but to depend on therapy to prolong survival.
Recent advancement in the pancreatic cancer chemotherapy has led in survival rates of between 10 months and 11 months. However, in cases when tumors neither show indications of tumor reduction or progress, there is no successful treatment option. Pharmacyte’s proposed treatment may fill this unmet medical need for advanced pancreatic cancer patients. This opportunity and need encouraged Pharmacyte to redesign its clinical trial. Moreover, this study also intends to assess effect of the therapy on the progression and development of the pain related with pancreatic cancer.
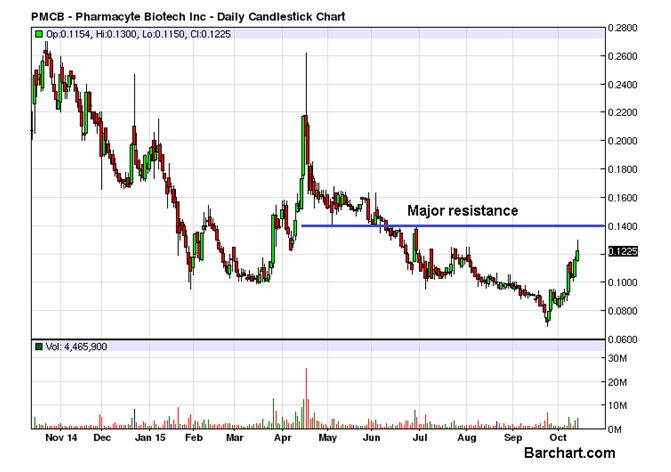
Pharmacyte Biotech Inc (OTCMKTS:PMCB) kept up rising for yet another day as it ended the last trading session with a gain of 5.79% but now the initial signs of an overstretching might have emerged. The volume of the day at 4.4 million was exactly double the daily average of 2.2 million, supportive of the upmove. On the other hand, the difference of almost 6% of the final closing price at $0.1225 from the day high of $0.13 indicated a bout of some serious profit booking at the higher levels. The proximity of the strong resistance at $0.14 can’t be comfortable for the bulls too.