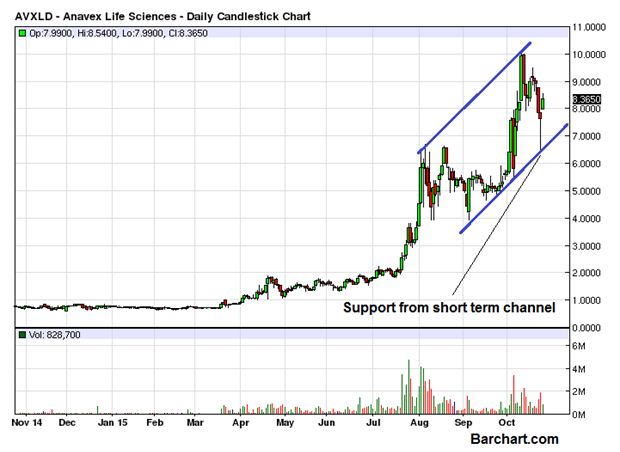
ANAVEX LIFE SCIENCES (OTCMKTS:AVXLD) bounced very sharply and closed at the end of the last trading session with a major gain of 9.35%. The volume of the day was not that encouraging as it was limited to 828,000 only, less than the daily average of 1.1 million. The stock has experienced a lot of volatility in the last few days as it rallied sharply only to be followed by an equally sharp correction retracing deeply. The entire price action of the last few months has been contained in a perfect channel as seen on the chart attached.
ANAVEX LIFE SCIENCES (OTCMKTS:AVXLD) a clinical-stage biopharmaceutical company formulating medications to treat Alzheimer’s , central nervous system diseases, pain and cancer, reported that it obtained approval to commence trading its common stock on the globally recognized NASDAQ Capital Market. The company’s common stock will commence to trade on NASDAQ platform under the ticker “AVXL” on October 28, 2015.
Accomplishment
Christopher U. Missling, PhD, the CEO and President of Anavex, said that it is a noteworthy achievement for company. As Anavex becomes a NASDAQ listed entity, the management is now able to demonstrate the overall progress to a broader audience. In increases the visibility of company’s capabilities and will create increased value for AVXL shareholders.
The uplisting on NASDAQ exchange comes at an important time as full PART A report and preliminary PART B Phase IIa clinical study results will be showcased at the CTAD conference to be held on November 7, 2015. Before commencing trading on NASDAQ platform on October 28, Anavex’s shares will continue to trade on OTC platform under the ticker “AVXLD.”
The highlights
Anavex Life is a publicly listed biopharmaceutical entity developing novel medications to treat Alzheimer’s and other central nervous system diseases. ANAVEX 2-73, the lead drug candidate, is in a Phase 2a study for Alzheimer’s. Preliminary positive report was showcased at AAIC 2015 and complete PART A report and initial results from PART B Phase IIa clinical study will be highlighted at CTAD next month.
The investigational drug ANAVEX 2-73 comes in the list of orally available drug candidates. It aims sigma-1 and muscarinic receptors to treat the fatal disease. The drug achieved success in Phase I clinical trial and demonstrated a clean data profile. Preclinical trials validated its potential to halt and reverse the course of AD. It also demonstrated neuro protective, anticonvulsant, anti-depressant and anti-amnesic properties indicating potential to cure additional CNS disorders.