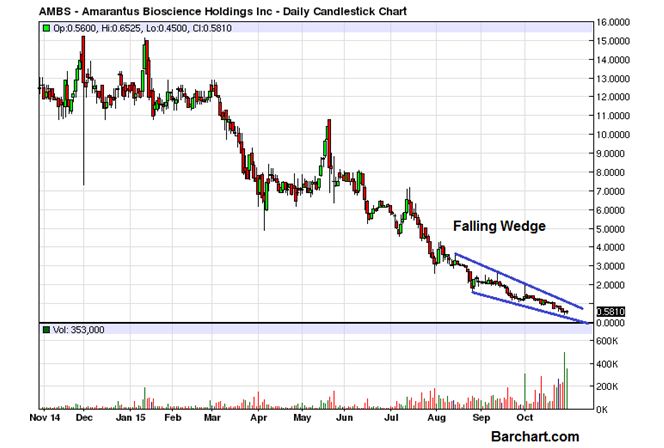
AMARANTUS BIOSCIENCE HOLDINGS, INC. (OTCBB:AMBS) opened slightly lower than the previous day’s close but managed to recover all the intraday losses and finally finished the last trading session with a minor gain of 0.17%. Generally, this kind of change in price would not be significant but when seen from the larger perspective, the gain is important as further evidence of the loss of bearish momentum. The chart attached clearly shows the Falling Wedge pattern in the last phase of the long term decline and can be a taken as a clear sign of the bears getting exhausted. A breakout above $1 can be taken as the initial signal of a turnaround.
AMARANTUS BIOSCIENCE HOLDINGS, INC. (OTCBB:AMBS) a biotechnology entity formulating diagnostic and therapeutic product candidates in neurology and orphan indications reported the issuance of Australian patent covering the application of Eltoprazine for the treatment of different mental and neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and other mental disorders.
The management comments
Gerald E. Commissiong, the CEO and President of Amarantus, said that this patent issuance for the application of Eltoprazine for the treatment of Alzheimer’s disease and Parkinson’s disease represents a noteworthy addition to the global intellectual property portfolio covering Eltoprazine. As the company reviews business development prospects, they believe the ongoing expansion of the patent position covering Eltoprazine will be significant.
Eltoprazine
Amarantus novel product Eltoprazine can be stated as a small molecule “5HT1A/1B” partial agonist currently in clinical advancement for the cure of Parkinson’s disease levodopa-resulted dyskinesia, adult attention deficit hyperactivity disorder and Alzheimer’s aggression. The drug has been evaluated in more than 680 human enrollments to date, and boasts a well-established safety profile. It was originally formulated by Solvay Pharmaceuticals for the cure of aggression. Upon Solvay’s merger completion with Abbott Pharmaceuticals, the respective medication was out-licensed to PsychoGenics, which further licensed Eltoprazine drug to Amarantus after successful proof-of-concept studies in adult ADHD and PD-LID.
The profile
Amarantus comes in the list of leading biotechnology firms developing diagnostics and treatments for diseases in the areas of orphan and neurology diseases. It possesses a sole international license to intellectual property rights related to Engineered Skin Substitute, an orphan medication selected autologous full thickness skin substitute product in advancement for the cure of adult severe burns set to enter Phase II clinical studies. Amarantus is currently assessing human clinical report from previously conducted trials in Congenital Giant Hairy Nevus and pediatric severe burns to support clinical advancement expansion into the respective areas.