A research report from ActiveMarketInsider.com at http://activemarketinsider.com/gequ-research-report/ has sent share prices of Global Equity International Inc through the roof today.
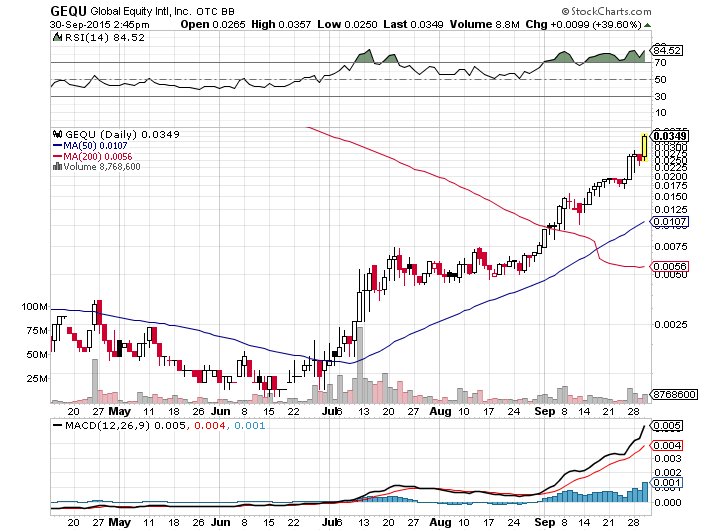
Shares of Global Equity International Inc (GEQU) are up by more than 39 percent today on heavy trading volume of more than 8.5M shares. Global Equity International Inc (GEQU) opened at $0.0265 and soon went on to hit a high of $0.0357.
Earlier on September 21st, GEQU through Its fully owned subsidiary Global Equity Partners Plc., Signed an agreement with a Reputable Italian Automotive Parts Manufacturer, International FIM SRL. It’s interesting to see such a late positive reaction from the trading and investing community.
In the press release, Peter Smith, CEO of GEP, said, “We at GEP believe that a quality client such as International FIM SRL with such an impressive client base and a long established business history will bring a plethora of value to our Company’s profile. We are confident that we can introduce International FIM to the appropriate funding that they require within the next 90 days through our vast network of financial institutions, hedge funds, wealth management offices and Sovereign wealth family offices in Europe and the Middle East.”
Let’s take a look at the chart below.
Since end of June, the stock prices have been appreciating gradually. As stated in our June 30, 2015 10Q filing, on August 6, 2015, the Company finally managed to rid itself of all convertible debt and does not intend to take on any further convertible debt in the foreseeable future.
It will be interesting to find out what really is fueling this penny stock to new highs. In any case, be sure to do your own due diligence and weigh out the risks before putting any money on the line.